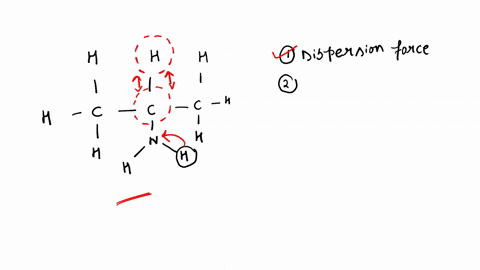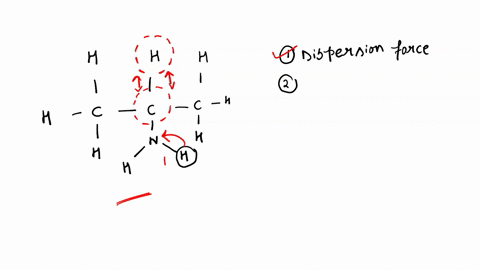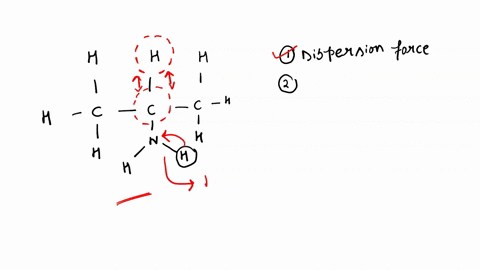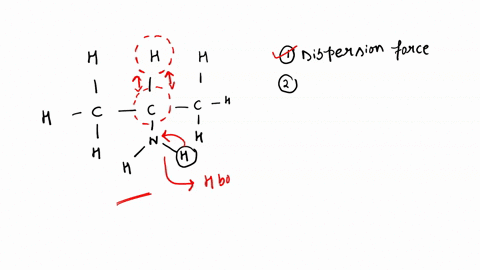
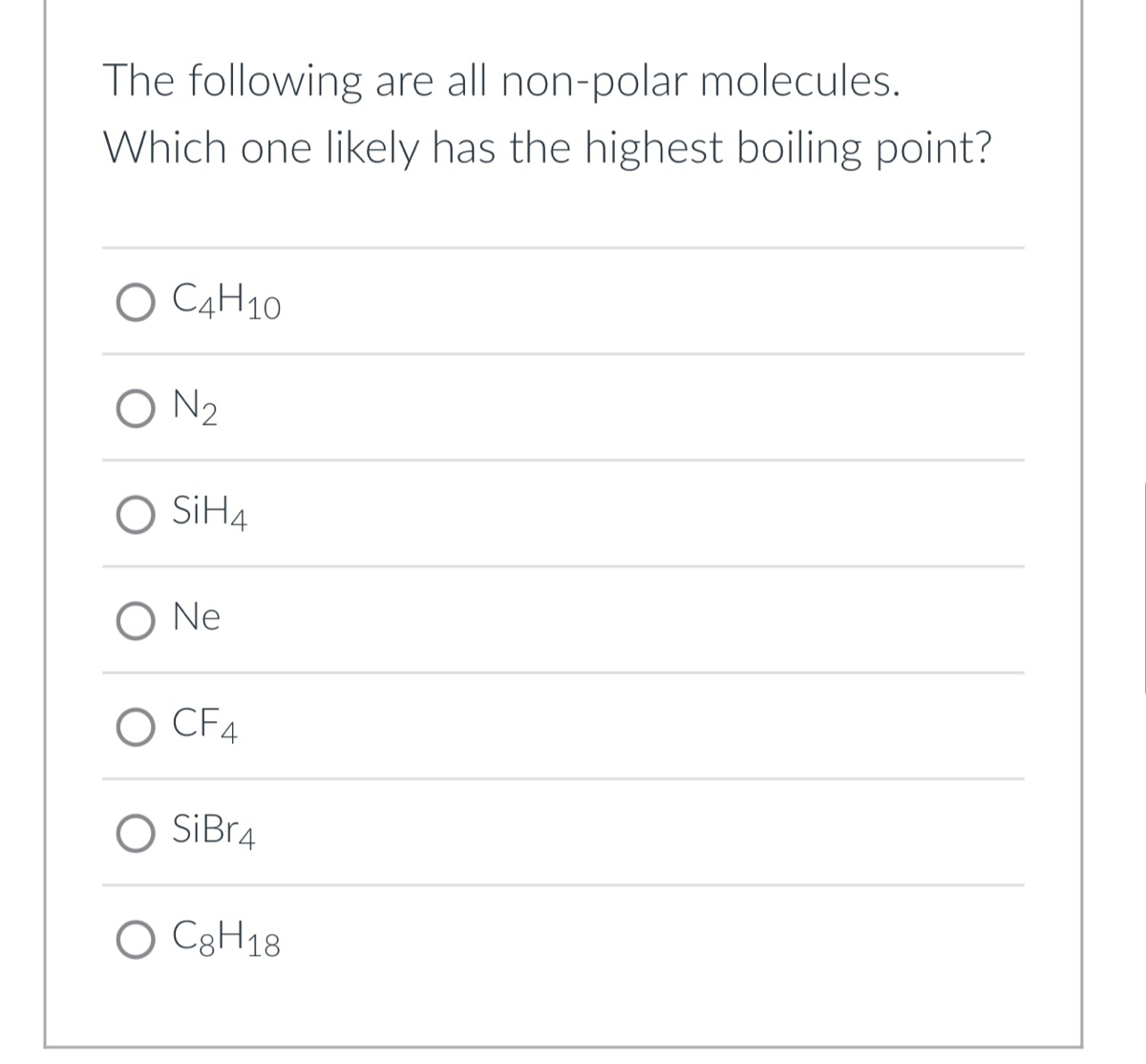
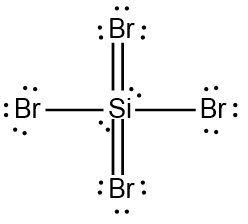
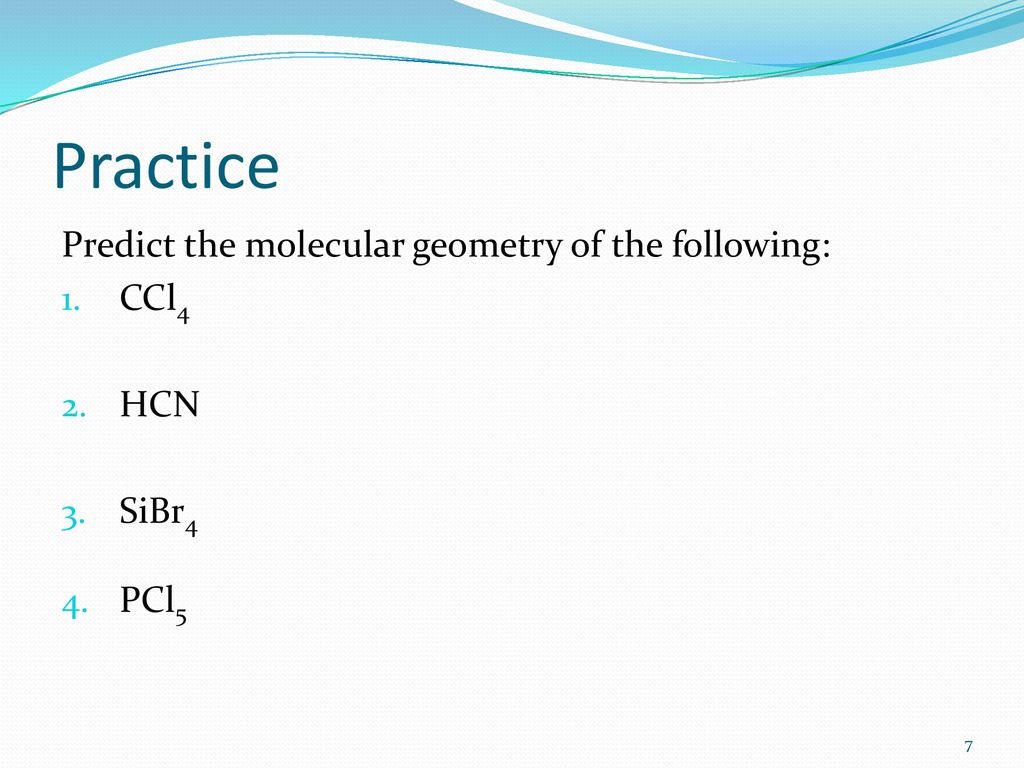
SOLVED: '(a) The stronger hydrogen bonding force in SiBr4 contributes to its higher boiling point.(b) The stronger dipole-dipole interaction force in SiBr4 causes its higher boiling point.(c) The stronger London dispersion force

Bonding Click to start Question 1 Which compound contains ionic bonds? Ethanoic acid, CH 3 COOH Dichloroethane, CH 2 Cl 2 Silicon tetrabromide,SiBr ppt download

Bonding Click to start Question 1 Which compound contains ionic bonds? Ethanoic acid, CH 3 COOH Dichloroethane, CH 2 Cl 2 Silicon tetrabromide,SiBr ppt download
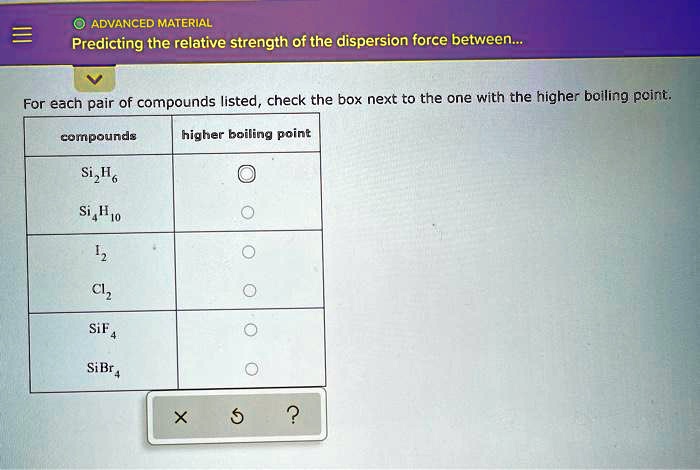
SOLVED: ADVANCED MATERIAL Predicting the relative strength of the dispersion force between For each pair of compounds listed check the box next to the one with the higher boiling pcint. compounds higher

SOLVED: From the list of substances below, select those for which dipole-dipole forces contribute to the attraction between molecules in the gas or liquid phase: You may select more than one substance.

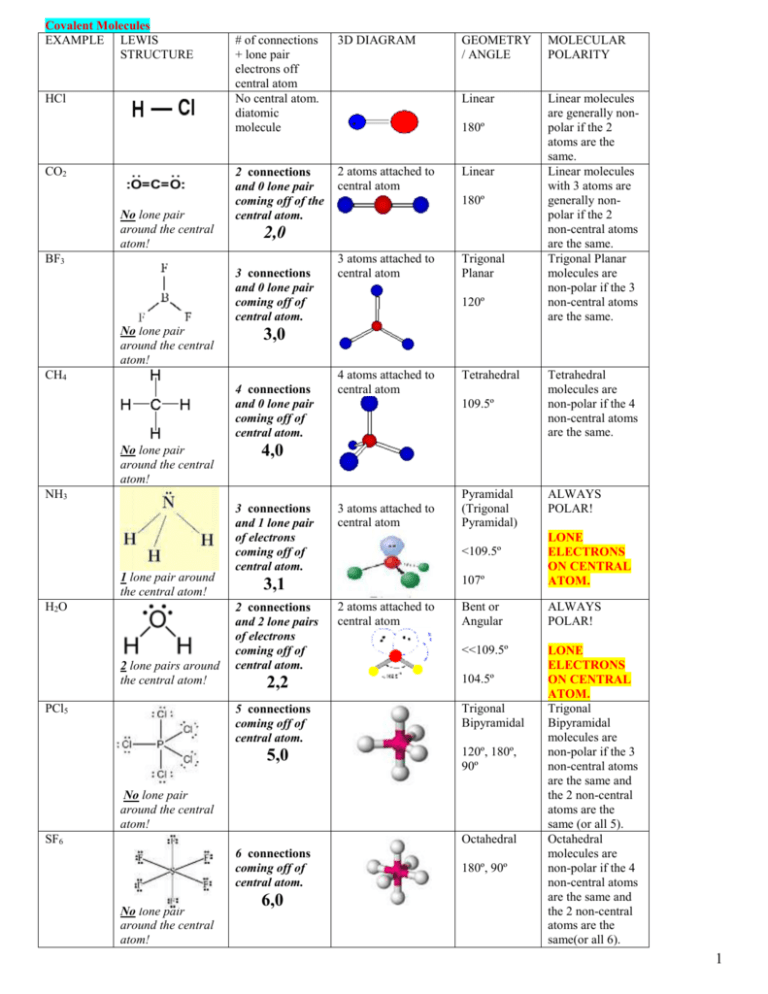
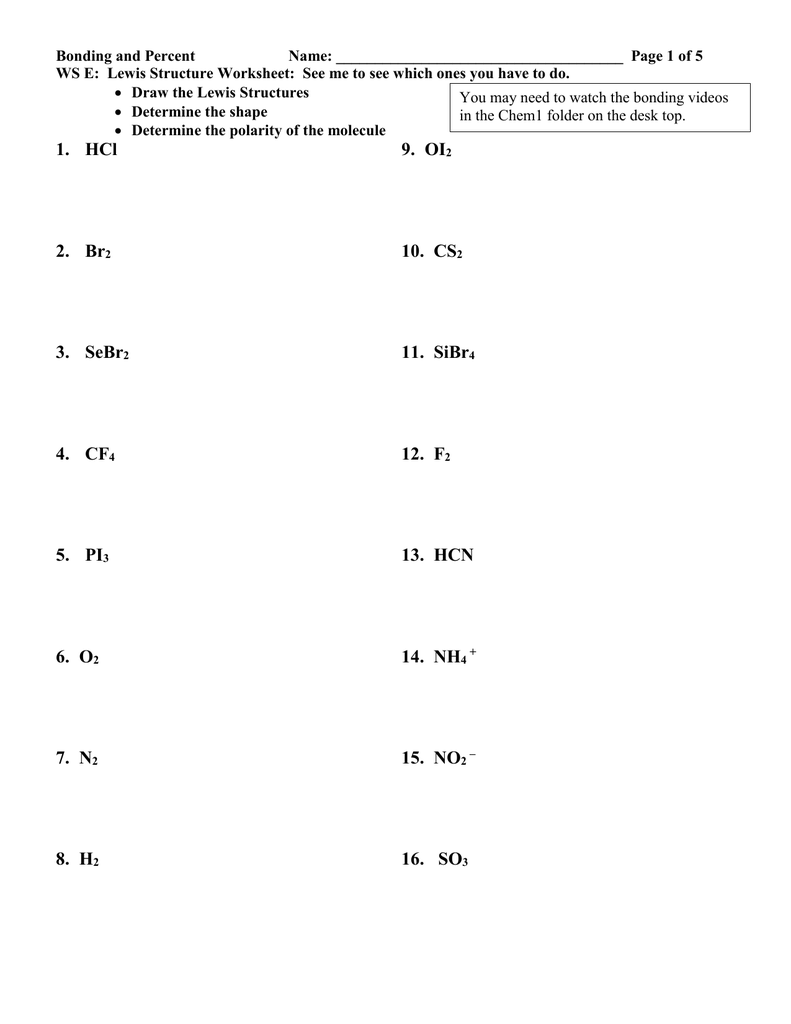
SOLVED: Review TOr 10.1 -11.4 Atoms share electrons in a covalent bond. What is true about covalent bonding? Select any that apply. Electrons are always evenly shared between the atoms in a

Intermolecular forces – dipole – dipole forces Lesson Objectives: To describe the interaction of molecules by permanent dipole – dipole To compare dipole. - ppt download
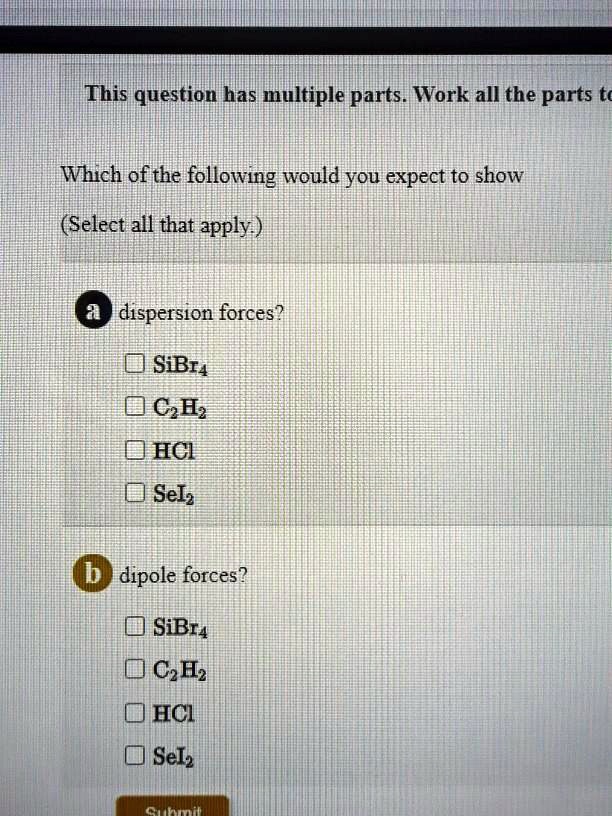
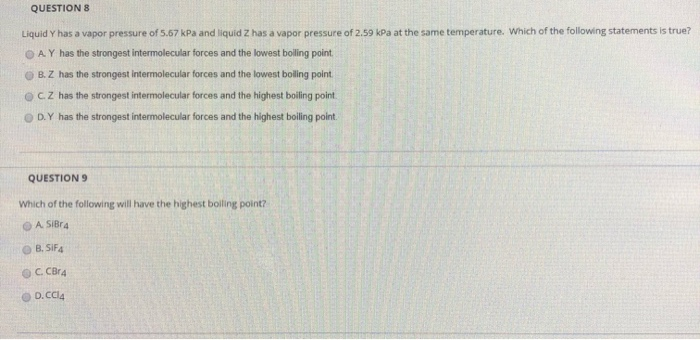
SOLVED: This question has multiple parts. Work all the parts Which of the following would you expect to show (Select all that apply ) dispersion forces? 0 SiBr4 0CH, 0HCI 0 Selz