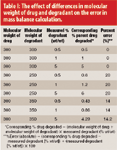
Assessing mass balance in pharmaceutical drug products: New insights into an old topic - ScienceDirect

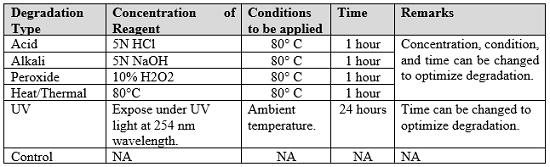
Development and Validation of Stability Indicating Rapid RP-LC Method for Determination of Process and Degradation Related Impurities of Apremilast, an Anti-Inflammatory Drug

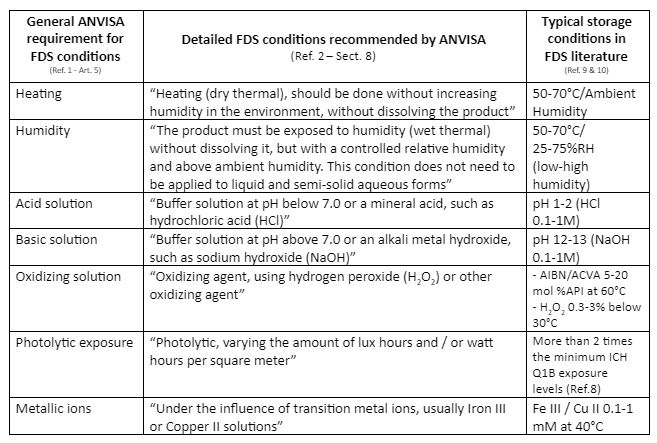
Impact from the Recent Issuance of ANVISA Resolution RDC-53/2015 on Pharmaceutical Small Molecule Forced Degradation Study Requirements | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

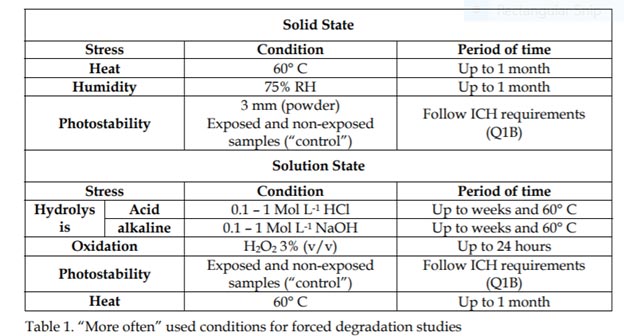
Forced degradation behavior of two-drug combinations: Isolation and characterization of major degradation products by LC-MS - ScienceDirect

Impact from the Recent Issuance of ANVISA Resolution RDC-53/2015 on Pharmaceutical Small Molecule Forced Degradation Study Requirements | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology