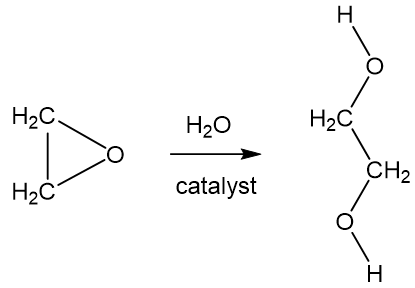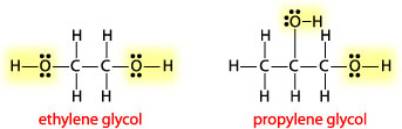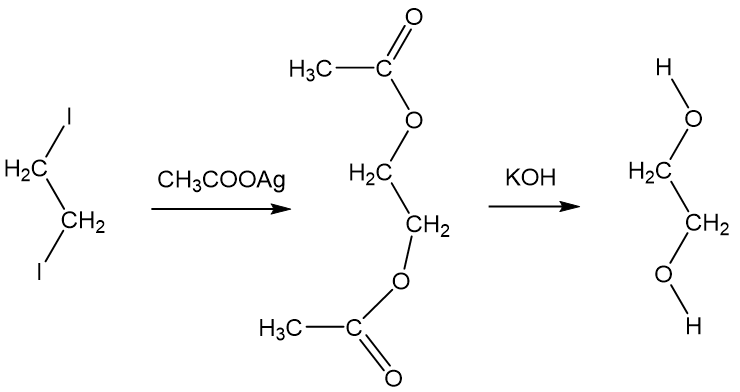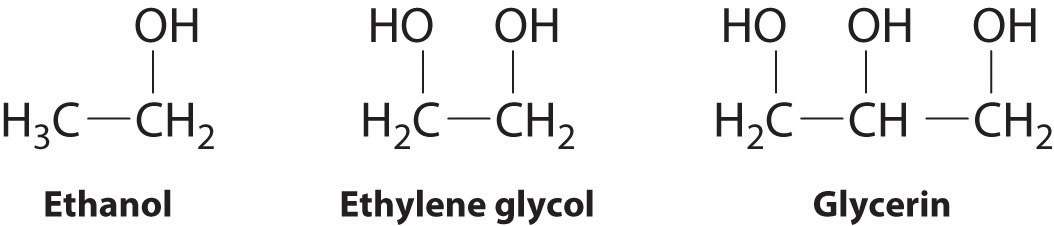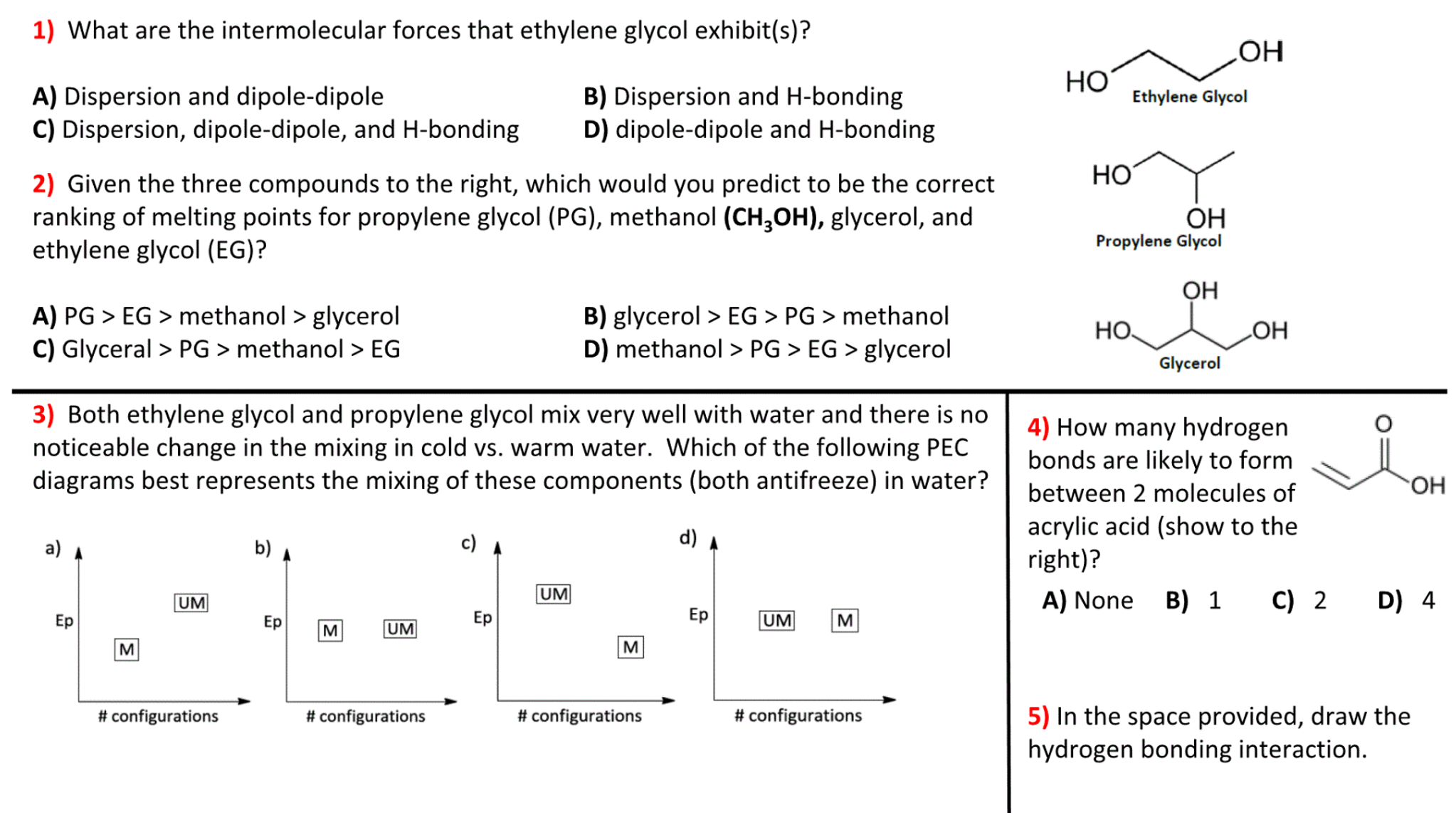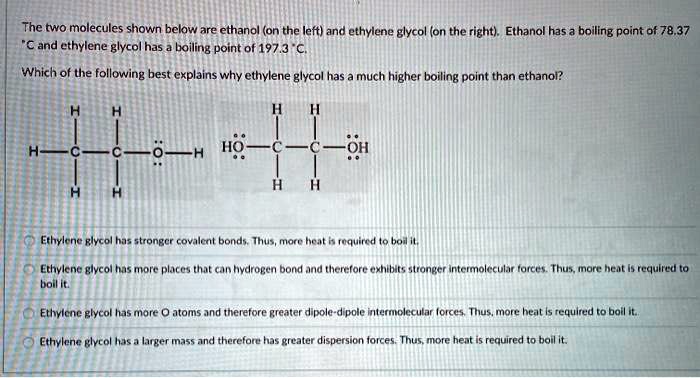
SOLVED: The two molecules shown bclw are ethanol (on the left) and ethylene glycol on the right) Ethanol has a bolling point of 78.37 C and cthylene glycol has boiling point of

SOLVED: LIQUIDS: Intermolecular Forces Acetone Acetonitrile Nitromethane Compound CzHsO CzHzN CHzNOz (nail polish remover) (used as a solvent) (drag racing fuel) H :0: H :0: Structural H CC-H H C-C==N: HC-N-o: formula

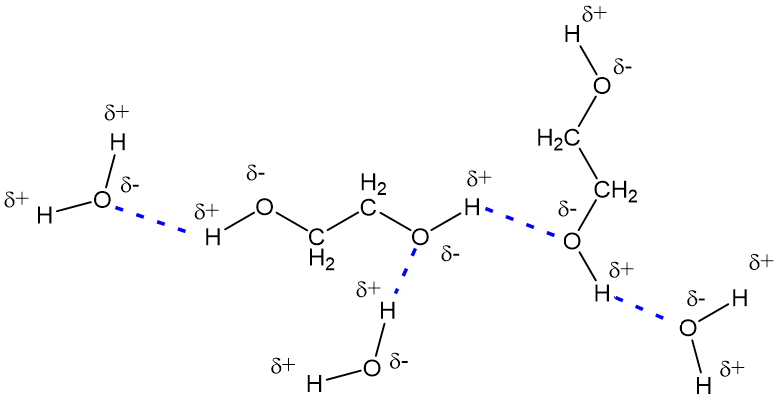
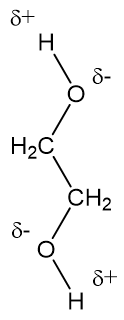
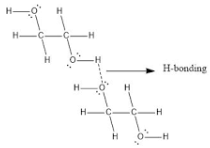
Ethylene glycol, HOCH_2CH_2OH, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Explain. | Homework.Study.com

Contact angles of water, diiodomethane, and ethylene glycol (a) and... | Download Scientific Diagram
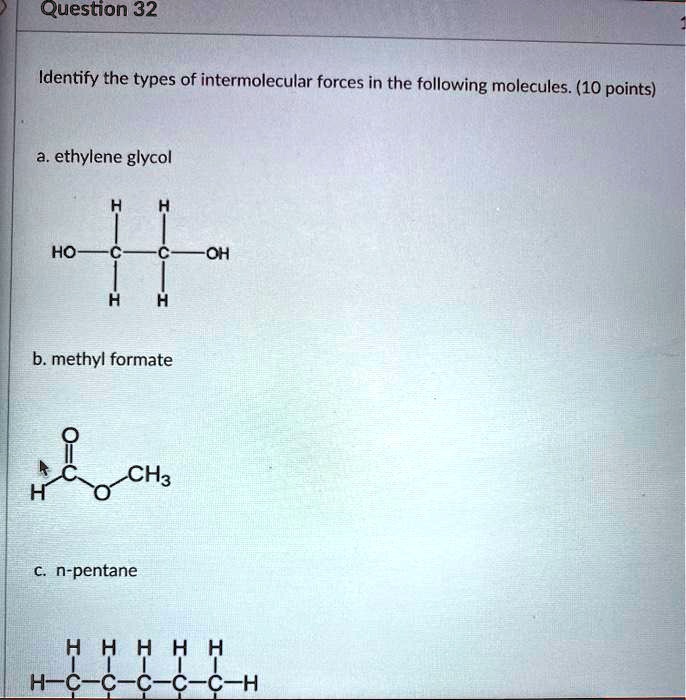
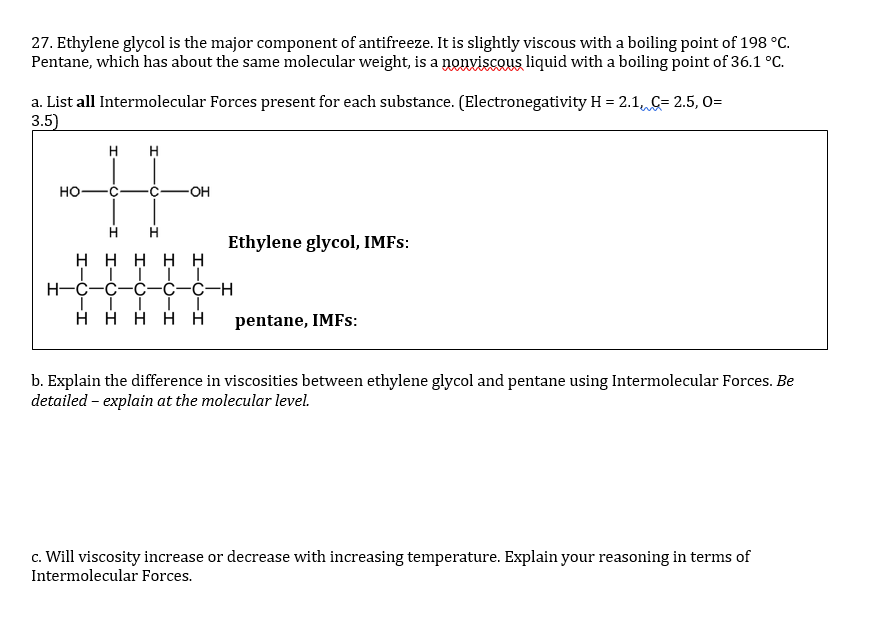
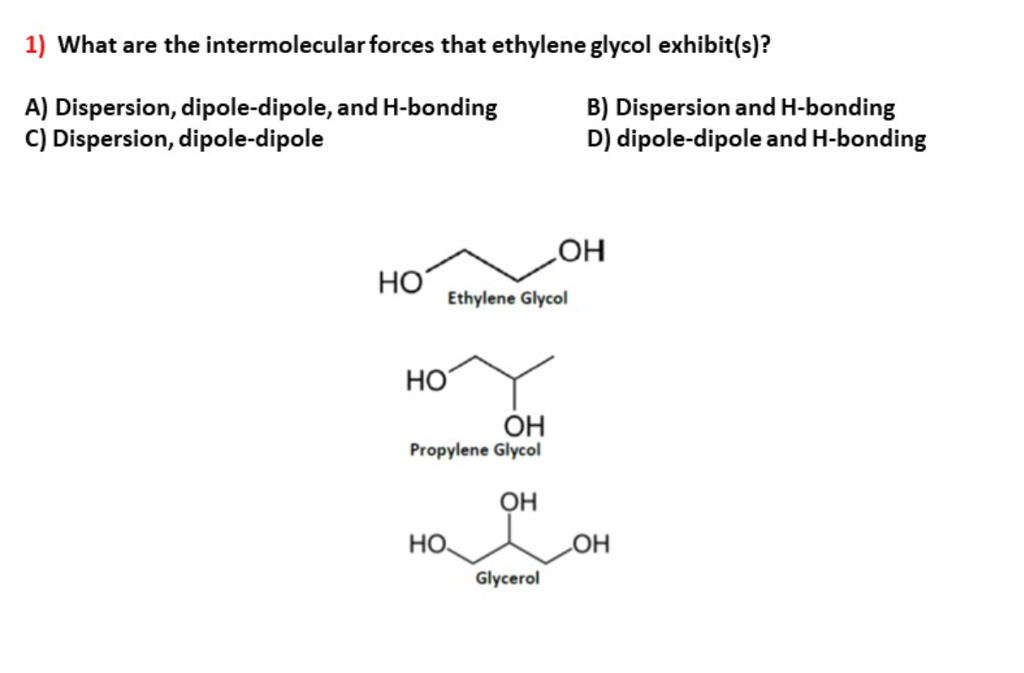
SOLVED: Question 32 Identify the types of intermolecular forces in the following molecules: (10 points) ethylene glycol HO OH b. methyl formate CH3 n-pentane H H H H H H-C-C-C-C-C-H

Ethylene glycol, HOCH_2CH_2OH, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Explain. | Homework.Study.com

Ethylene glycol, HOCH_2CH_2OH, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Explain. | Homework.Study.com
![College Level: Inorganic Chemistry] Intermolecular Forces: Determining the solubility of Acetone/Urea in both Ethylene Glycol and O-Dichlorobenzene : r/HomeworkHelp College Level: Inorganic Chemistry] Intermolecular Forces: Determining the solubility of Acetone/Urea in both Ethylene Glycol and O-Dichlorobenzene : r/HomeworkHelp](https://i.redd.it/jdzmwtz099e41.jpg)