
Risk-Based Biologics: CMC Flexibilities in the EU Regulatory System - BioProcess InternationalBioProcess International
The pharmaceutical development guidelines suggested by ICH, US FDA and... | Download Scientific Diagram
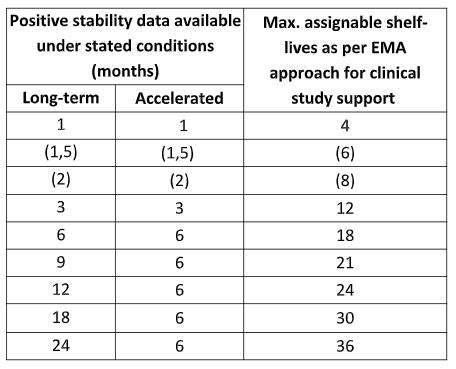
Stability Testing Study Design and Data Evaluation to Support a Clinical Study in the European Union: Part 2 - StabilityHub

PVpharm on the EMA course: Mandatory use of ISO/ICH E2B(R3) Individual Case Safety Reporting in the EU – PV PHARM

EMA-consult: ICH-richtlijn E8 (R1) over algemene overwegingen voor klinische onderzoeken - Stap b - Acron

EMA released for public consultation the draft ICH guideline M10 on bioanalytical method validation | EPTRI










.jpg)


